INTRODUCTION
Nano means one billionth that means 10^-9 times in scientific notation. Have you ever thought how small it is? Avg human height is around 1.5-2m, size of ants are about 2mm, the diameter of a human hair is around 100mm and size of our DNA is around 2nm that means it is 10^-9 times smaller than average human height. To imagine how small is one-billionth let’s go on the other side and see how big an object would be if we are one billionth time larger than the humans. The diameter of the sun is about one billionth times larger than a human. That’s pretty big. So our DNA is as small as humans as humans are from the sun.
What are nanomaterials?? What is its importance? Where are they used? Let’s dive into the world of smallness!!!
Nanomaterials include a broad class of materials, which has at least one dimension less than 100nm. Depending on their shape, they can be 0-D, 1-D, 2-D or 3-D. You may be thinking what this small piece of material can do?? Nanomaterials have an extensive range of applications. The importance of these materials was realized when it was found that size can influence the physicochemical properties of a substance. Nanoparticles have biomedical, environmental, agricultural and industrial based applications.
Nanoparticles are composed of 3 layers-
The Surface Layer- It may be functionalized with a variety of small molecules, metal ions, surfactants and polymers.
The Shell Layer- It is a chemically different material from the core in all aspects.
The Core- It is the central portion of the nanoparticle and usually referred to as nanoparticle itself.
These materials got immense interest from researchers in multidisciplinary fields due to their exceptional characteristics.
CLASSIFICATION OF NANOPARTICLES
Based on the physical and chemical characteristics, some of the well-known classes of NPs are-
FULLERENES- It contains nanomaterials that are made up of globular hollow cage such as allotropic forms of carbon. They have properties like electrical conductivity, high strength, structure, electron affinity and versatility. They possess pentagonal and hexagonal carbon units, while each carbon is sp2 hybridized. The structure of C-60 is called Buckminsterfullerene
CARBON NANOTUBES(CNTs)- They have elongated, tubular structure, 1-2nm in diameter. They structurally resemble graphite sheets rolling upon itself, which can have single double and many walls and therefore are named as single-walled (SWNTs), double-walled (DWNTs) and multi-walled carbon nanotubes (MWNTs) respectively. They are widely synthesized by decomposition of carbon, especially atomic carbons, vaporized from graphite by laser or by an electric arc to metal particles. Chemical Vapour Deposition (CVD) technique is also used to synthesize CNTs. They can be used as fillers, efficient gas absorbents and as a support medium for different inorganic and organic catalysts.
They are purely made up of metal precursors. Due to Localized Surface Plasmon Resonance (LSPR) characteristic, they possess unique optoelectrical properties. Due to excellent optical properties, they find their application in various research areas. For example, gold nanoparticles are used to coat the sample before analyzing in SEM.
They are inorganic, nonmetallic solids, synthesized via heat and continuous cooling. They are made up of oxides, carbides, carbonates and phosphates. They can be found in amorphous, polycrystalline, dense, porous or hollow forms. They found their application in catalysis, photocatalysis, photodegradation of dyes and imaging application.
They possess wide band gaps and therefore show significant alteration in their properties with bandgap tuning. They are used in photocatalysis, photo optics and electronic devices. Some of the examples of semiconductor NPs are GaN, GaP, InP, InAs.
They are organic-based NPs, mostly nanospheres and nanocapsules in shape. They are readily functionalized and therefore have a wide range of applications.
They contain liquid moieties and are effectively used in many biomedical applications. They are generally spheres with diameters ranging from 10 to 1000nm. They have a solid core made of lipid, and a matrix contains soluble lipophilic molecules.
SYNTHESIS OF NPs
There are various methods used for the synthesis of NPs, which are broadly classified into two main classes-
Top-down routes are included in the typical solid-state processing of the materials. It is based on bulk materials and makes it smaller, thus using physical processes like crushing, milling and grinding to break large particles. It is a destructive approach, and it is not suitable for preparing uniformly shaped materials. The biggest drawback in this approach is the imperfections of the surface structure, which has a significant impact on physical properties and surface chemistry of nanoparticles. Examples of this approach include grinding/milling, CVD, PVD and other decomposition techniques.
As the name suggests, it refers to the build-up of materials from the bottom: atom by atom, molecule by molecule or cluster by cluster. They are more often used for preparing most of the nanoscale materials which have the ability to generate uniform size, shape and distribution. It effectively covers chemical synthesis and precisely controls the reaction to inhibit further particle growth. Examples are sedimentation and reduction techniques. It includes sol-gel, green synthesis, spinning and biochemical synthesis.
CHARACTERIZATION OF NPs
Analysis of different physicochemical properties of NPs is done using various characterization techniques. It includes techniques such as X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), Infrared (IR), SEM, TEM and particle size analysis.
Morphology always influences most of the properties of the NPs. Microscopic techniques are used for characterization for morphological studies such as a polarized optical microscope, SEM and TEM.
SEM technique is based on electron scanning principle. It uses a focused beam of high energy electrons to generate a variety of signals at the surface of solid specimens. It is not only used to study the morphology of nanomaterials, but also the dispersion of NPs in the bulk or matrix.
TEM is based on electron transmission principle so that it can provide information on bulk material from very low to higher magnification. In TEM a high energy beam of electrons is shone through a skinny sample. This technique is used to study different morphologies of gold NPs. It also provides essential information about two or more layer materials.
Structural characteristics are of primary importance to study the composition and nature of bonding materials. It provides diverse information about the bulk properties of the subject material. XRD, Energy dispersive X-ray (EDX), XPS, IR, Raman and BET are the techniques used to study the structural properties of NPs.
XRD is one of the most used characterization techniques to disclose the structural properties of NPs. Crystallinity and phases of nanoparticles can be determined using this technique. Particle size can also be determined by using this technique. It worked well in identification of both single and multiphase NPs.
EDX is usually fixed with field emission-SEM or TEM device is widely used to know about the elemental composition with a rough idea of per cent weight. Nanoparticles comprise constituent elements, and each of them emits characteristic energy X-rays by electron beam eradication.
XPS is one of the most sensitive techniques used to determine the exact elemental ratio and exact bonding nature of elements in nanoparticles materials. It is a surface-sensitive technique used in-depth profiling studies to know the overall composition and the compositional variation with depth.
Size of the particle can be estimated by using SEM, TEM, XRD and dynamic light scattering (DLS). Zeta potential size analyzer/DLS can be used to find the size of NPs at a deficient level.
NTA is another new and exclusive technique which allows us to find the size distribution profile of NPs with a diameter ranging from 10 to 1000nm in a liquid medium. By using this technique, we can visualize and analyze the NPs in a liquid medium that relates the Brownian motion rate to particle size. It can be helpful in biological systems such as protein and DNA.
NPs have large surface areas, so it offers excellent room for various applications. BET is the most used technique to determine the surface area of nanoparticles material. Principle of this technique is adsorption and desorption and Brunauer-Emmett-Teller (BET) theorem.
Optical properties are of great concern in photocatalytic applications. These characterizations are based on Beer-lambert law and basic light principles. The techniques used to give information about absorption, luminescence and phosphorescence properties of NPs. The optical properties of NPs materials can be studied by well-known equipment like Ultraviolet-visible, photoluminescence and the ellipsometer.
PHYSICOCHEMICAL PROPERTIES OF NPs
So it’s all about the size, isn’t it? Yes and no. When a material becomes a nanomaterial is not so simple. A nanomaterial may have different properties compared to the same substance in bulk form. That means that a material could change when it goes from bulk to nanoform, but at what size that happens varies depending on the substance.Nanoparticles are used in various applications due to their unique properties such as large surface area, strength, optically active and chemically reactive.
The optical and electronic properties of nanoparticles are dependent on each other. For example, gold colloidal nanoparticles are the reason for the rusty colours seen in blemished glass windows, while Ag NPs are typically yellow. The free electrons on the surface of nanomaterials are free to move across the material. The mean free path of Ag and gold is ~50nm, which is greater than the NPs size of these materials. Therefore, no scattering is expected from the bulk, when light interacts. Instead, they set into a standing resonance condition, which is responsible for LSPR in the NPs.
There is a class of nanoparticles known as magnetic nanoparticles that can be manipulated using magnetic fields. Such particles consist of two components- a magnetic material and chemical component that has functionality. These types of materials have a wide range of applications which includes heterogeneous and homogeneous catalysis, biomedicine, magnetic fluids, MRI and also in water decontamination. Magnetic properties of NPs dominate when its size is less than the critical value, i.e. 10-20nm. The reason for these magnetic properties is the uneven electronic distribution in NPs.
To know the exact mechanical nature of NPs different mechanical parameters such as elastic modulus, hardness, stress and strain, adhesion and friction are surveyed. Due to distant mechanical properties of NPs, it finds its application in fields like tribology, surface engineering, nanofabrication and nanomanufacturing. NPs shows different mechanical properties as compared to microparticles and their bulk materials.
It is well known that metals have better thermal conductivities than that of fluids. Same is the case of NPs. Thermal conductivity of copper is much higher than water and engine oil. Thermal conductivity of fluids can be increased by dispersing solid particles in them. Using the same way nanofluids are produced which have nanometric scales solid particles dispersed into a liquid such as water, ethylene glycol or oils. They are expected to exhibit superior properties relative to those of conventional heat transfer fluids and fluids containing microscopic solid particles. As heat transfer takes place at the surface of the particles, it is better to use the particles with large surface area, and it also increases the stability suspension.
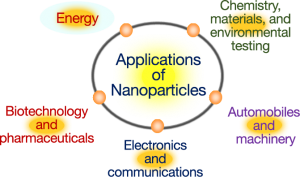
APPLICATIONS
As discussed above the nanoparticles have various unique properties. Due to their properties, they find their applications in multiple fields, including drugs, medication, manufacturing, electronics, multiple industries and also in the environment.
Nano-sized inorganic particles have unique, physical and chemical properties. They are an essential material in the development of various nanodevices which can be used in multiple physical, biological, biomedical and pharmaceutical applications. Particles of an iron oxide such as magnetite (Fe3O4) or its oxides from maghemite (Fe2O3) are used in biomedical applications. Polyethene oxide (PEO) and polylactic acid (PLA) NPs have been revealed as up-and-coming systems for the intravenous administration of drugs. Biomedical applications require NPs with high magnetization value, a size smaller than 100nm and a narrow particle size distribution. Most of the semiconductor and metal NPs have immense potential cancer diagnosis and therapy.
Image shows the bamboo-like structure of nitrogen-doped carbon nanotubes for the treatment of cancer.
In specific applications within the medical, commercial and ecological sectors manufacturing NPs are used which show physicochemical characteristics that induce unique electrical, mechanical, optical and imaging properties. Nanotechnology is used in various industries, including food processing and packaging. The unique plasmon absorbance features of the noble metals NPs have been used for a wide variety of applications including chemical sensors and biosensors.
Nanomaterials are also used in some environmental applications like green chemistry, pollution prevention, the recommendation of contaminated materials and sensors for ecological stages.
NPs such as metallic NPs, organic electronic molecules, CNTs and ceramic NPs are expected to flow as a mass production process for new types of electronic equipment.
NPs can also offer applications in mechanical industries, especially in coating, lubricants and adhesive applications. Its mechanical strength can be used to produce mechanically more reliable nanodevices.
CONCLUSION
Nanomaterials are no doubt the future of technology, being the smallest material they have a wide range of applications due to their unique physical and chemical properties. Due to their small size, NPs have a large surface area which also makes them suitable candidates for many applications. Even at that size, optical properties dominate, which further increase their importance in photocatalytic applications. Though NPs are used for various applications, still they have some health hazard concerns due to their uncontrollable use and discharge to the natural environment, which should be considered to make the use of NPs more convenient and environmentally friendly.
WONDER, THINK, CREATE!!!
Keep Learning!, Keep Growing!
Team CEV